
Why choose zeta farmaceutici?
- Business, economic, and production stability
- Knowledge of the production processes and pharmaceutical quality
- Support and experience in development of projects for large pharma companies
Which services we provide
- Development and production
- Support with analytics and regulatory compliance
- Pharmaceutical technology transfer
- Sampling for clinical trials
For which product categories
We are experts in the development and production of pharmaceuticals but we also have consolidated knowhow in the sector of medical devices, cosmetics and dietary supplements, again with a pharmaceutical approach and for large volumes.
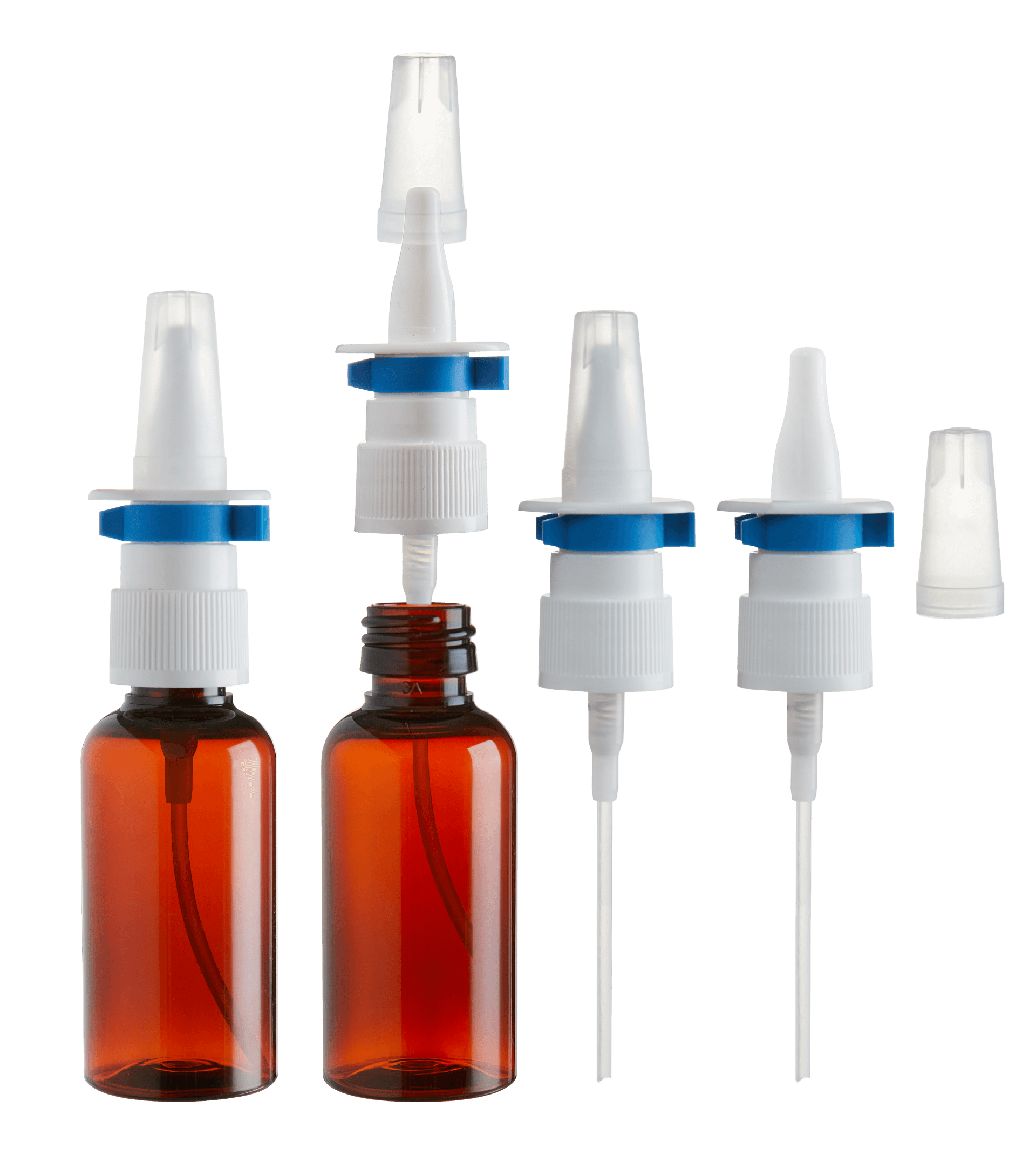
These are pharmaceuticals formats that require great expertise to ensure the medicine is administered accurately. Knowledge of the active ingredient, packaging and administering system are the basis for correct development and production of the product.

This pharmaceutical format requires perfect knowledge of the characteristics and behaviour of the liquid containing the active ingredient, both at the dosing stage and inside the stick pack. Furthermore, the project’s success depends on expertise in the world of flavouring.

We are the only company able to use this kind of packaging for pharmaceuticals. We developed it in collaboration with the top primary packaging manufacturer in the sector in order to guarantee the long-term stability of the medicine. The process takes place in environments with strictly controlled humidity and temperature parameters.

Nous produisons ces formes pharmaceutiques en plusieurs types de flacons, différents par leur format, leur matériau et leur système de fermeture, auxquels sont ajoutés un gobelet, une cuillère ou une seringue de dosage. Parallèlement aux formes destinées à une administration orale, nous produisons des suspensions à usage rectal en lavements et microlavements.
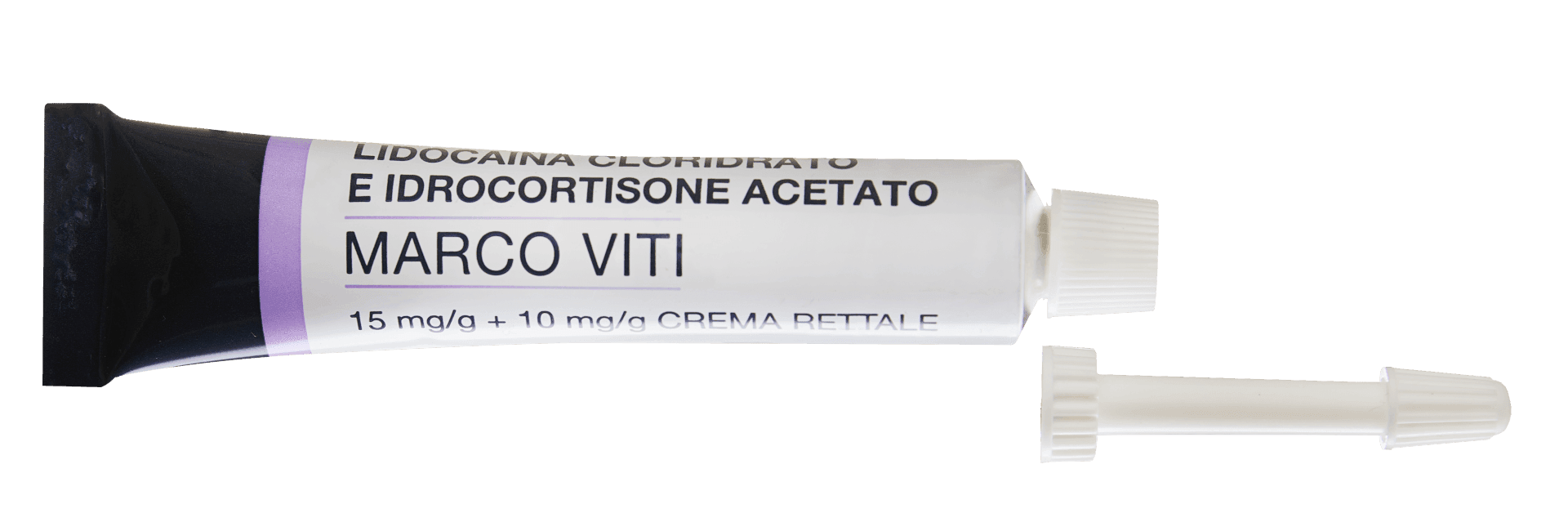
We make products using cortisone-based active ingredients in liquids (drops, sprays, enemas) or semi-solids (creams and ointments) in AIFA-authorised environments inspected by the major pharmaceutical companies.

These pharmaceutical active ingredients are a particularly delicate category from a point of view of production, quality and, above all, regulatory compliance. Oral administration in drops requires great competence in terms of safety and pharmacological principles.

Decades of research, development and realisation of yeast-based products and food-grade probiotics have brought us AIFA (Italian Medicines Agency) authorisation for the production of medicines containing bacterial and non-bacterial microorganisms such as Saccharomyces Boulardii yeast. As well as guaranteeing the quality of the medicine, we also had to put correct cleaning and safety protocols in place for the environments and operators.


© ZETA FARMACEUTICI S.P.A. | P.Iva IT00330790247 | Privacy Policy | Company Info | Health supervision | Whistleblowing | Contacts
 health supervision
health supervision